Patient Highlights
- clonoSEQ Tracking (MRD) testing revealed early signs of relapse post-transplant.
- Physician had opportunity to prepare options for follow-up treatment, allowing him to act quickly once relapse was confirmed.
Patient History
- Diagnosed with B-acute lymphoblastic leukemia
- Decision to proceed to allotransplant was made after low-level MRD remained following several rounds of chemotherapy
- Patient received unrelated donor transplant in early 2014. Patient’s chimerism was dropping post-transplant but patient remained MRD-negative by flow. NGS detected MRD+ in April 2015. Five months later, MRD+ detected was by flow
- Patient recurred in September 2015 and was enrolled in CAR-T therapy trial in November 2015
Physician's Perspective
“When a patient has morphologic relapse, you feel anxious and need to act. Knowing that a patient is relapsing by detecting disease at a much lower level gives us a window of time to prepare instead of having to act immediately when a kid comes in with packed marrow and a fever.
Once a positive MRD test comes back, I increase the frequency of testing in order to stay on top of disease. In the case of this patient, upon the first positive MRD test, we banked T cells in case the patient was eligible for an immunotherapy trial. Once he relapsed, I enrolled him immediately in a CAR-T trial, and since that treatment two years ago he has been in remission.
People say that there is no advantage to detecting disease earlier, but that’s changing. We have new transplant modalities, immunotherapies like blinatumumab, and many CAR trials available, so detecting disease early may be advantageous to the patient.”*
*Clinician has received compensation to participate in advisory meetings sponsored by Adaptive. Clinician’s research has also been supported, in part, via product grants.
Use of the clonoSEQ Assay
-

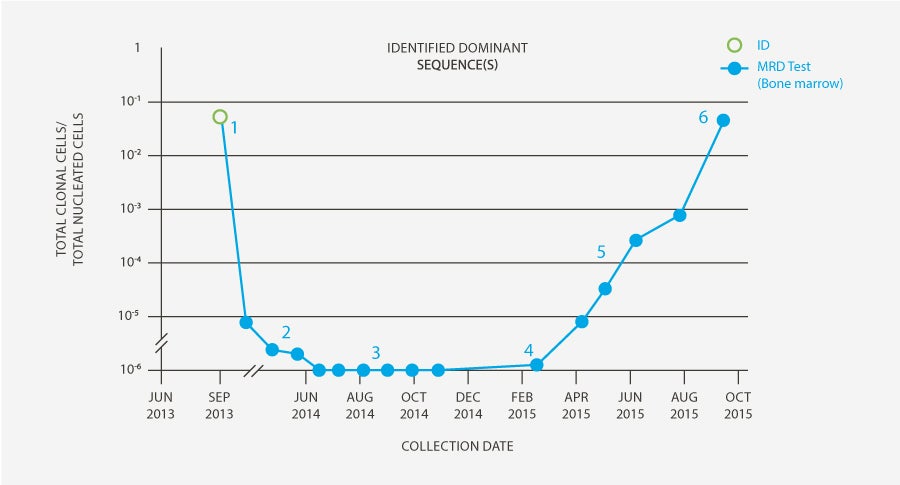
Physician ordered Clonality (ID) test so that he would have freedom to use clonoSEQ MRD testing in the future if desired.
-

MRD was detectable at 30, 60 and 90 days post-transplant.
-

The patient was clonoSEQ MRD-negative in the bone marrow at 4, 5, 6, 7, 8, and 9 months post-transplant.
-

One year post-transplant, MRD was detected by clonoSEQ (1 leukemic molecule / million cells). The patient remained MRD-negative by flow. Assessment of chimerism revealed that patient had lost his graft.
-

Physician made decision to conduct monthly clonoSEQ MRD evaluation. The patient’s MRD continued to rise month-over-month. Options for further treatment discussed; decision made to bank T cells for potential CAR-T treatment.
-

MRD continued to increase, and morphologic relapse was observed (7 months after initial positive MRD test). Patient received CAR-T therapy in November 2015 as part of a trial and has been in remission for 2 years.
*This case study was based off results generated from an earlier version of the clonoSEQ Assay.
Intended Use:
The clonoSEQ Assay is an in vitro diagnostic that uses multiplex polymerase chain reaction (PCR) and next-generation sequencing (NGS) to identify and quantify rearranged IgH (VDJ), IgH (DJ), IgK and IgL receptor gene sequences, as well as translocated BCL1/IgH (J) and BCL2/IgH (J) sequences in DNA extracted from bone marrow from patients with B-cell acute lymphoblastic leukemia (ALL) or multiple myeloma (MM), and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL).
The clonoSEQ Assay measures minimal residual disease (MRD) to monitor changes in burden of disease during and after treatment. The test is indicated for use by qualified healthcare professionals in accordance with professional guidelines for clinical decision-making and in conjunction with other clinicopathological features.
The clonoSEQ Assay is a single-site assay performed at Adaptive Biotechnologies Corporation in Seattle, Washington.
Special Conditions for Use:
- For in vitro diagnostic use.
- For prescription use only (Rx only).
Limitations:
ALL, MM, and CLL:
MRD values obtained with different assay methods may not be interchangeable due to differences in assay methods and reagent specificity. The results obtained from this assay should always be used in combination with the clinical examination, patient medical history, and other findings. The clonoSEQ Assay is for use with specimens collected in EDTA tubes. Results may vary according to sample time within the course of disease or by sampling site location. The assay may overestimate MRD frequencies near the limit of detection (LoD). The MRD frequency LoD varies based on the amount of gDNA that is tested and using lower gDNA input may prevent MRD detection at low frequencies. Sample processing and cell enrichment strategies may affect the measured MRD frequency. The volume and cellularity of sampled input material may affect the ability to detect low levels of disease. False positive or false negative results may occur for reasons including, but not limited to: contamination; technical and/or biological factors such as the type of rearrangement or the size of the junction region. The assay has been validated with the Illumina NextSeq500 and 550.
For CLL:
MRD is based on measurements of tumor cells detected in peripheral blood and/or bone marrow. However, patients may have significant residual disease in unassessed compartments and U-MRD in one compartment cannot fully rule out the presence of disease in the other compartment, for example, U-MRD in blood may not be the same in bone marrow. Therefore assessment of MRD in CLL should employ a multimodal approach including clinical examination, patient medical history, and other findings. Outcome for patients with MRD detectable in bone marrow but not in peripheral blood (PB-/BM+) may differ according to type of therapy. This assay is capable of monitoring specific tumor clonotypes. The association between MRD assessments and patient clinical status for the purpose of monitoring changes in disease (e.g., relapse, remission, stable disease) has not been demonstrated. The value of MRD in CLL for previously untreated or “watch and wait” patients is not established. CLL is a heterogeneous disease. MRD values and expectations for outcome may not be generalizable across treatments. Changes in MRD should be interpreted with caution when used to evaluate disease burden in therapies that have not been validated. Regardless of MRD status, cytogenetics play an independent role in patient risk status and its impact on PFS/OS.
For important information about the FDA-cleared uses of clonoSEQ including test limitations, please visit clonoSEQ.com/technical-summary.
 Back
Back