Patient Highlights
- Adult acute lymphoblastic leukemia (ALL) patient; Philadelphia chromosome (Ph)-negative with standard risk features
- Patient achieved minimal residual disease (MRD) negativity following induction + consolidation and proceeded to transplant
- 100 days post-transplant, clonoSEQ Tracking (MRD) test revealed low-level MRD in the bone marrow, which was then monitored and subsequently became detectable in the blood
- Clinician initiated blinatumomab* therapy with goal of achieving MRD negativity
Patient History
- Adult Ph-negative patient with normal karyotype, 70% blasts in bone marrow and immunophenotype consistent with B-ALL (CD34+, CD38+, HLA-DR+, CD10+, CD19+, CD20+, TDT+)
- Achieved CR following Linker induction regimen with rituximab but still had 0.3% MRD by flow cytometry
- After consolidation, achieved MRD-negative complete response (CR) by clonoSEQ and proceeded to matched unrelated donor transplant
- Evidence of low-level but rising clonoSEQ MRD post-transplant led first to immune suppression taper and eventually to initiation of blinatumomab
Physician's Perspective
“Many adult ALL patients achieve a CR after initial therapy but may have residual disease that puts them at risk of a relapse. With the FDA approval of blinatumomab, clinicians have a therapeutic option to address MRD positivity. MRD-directed therapy is an important advance for patients, but it also creates a need for a highly accurate, reliable and sensitive MRD testing method, which is why I use clonoSEQ to assess MRD prior to initiating blinatumomab.
For this patient, I used clonoSEQ to monitor MRD throughout treatment. The patient initially achieved MRD-negativity, but when clonoSEQ detected very low level residual disease in the bone marrow shortly following transplant, I began evaluating MRD in the blood as a means to monitor the patient more closely. When disease became detectable in the blood, I confirmed the rise in MRD with a repeat bone marrow and then initiated blinatumomab.** My goal for this patient was to reestablish MRD negativity at 1 in 1 million sensitivity; this is the same goal I strive for now when I initiate blinatumomab in post-induction MRD-positive patients in CR.
This patient responded to blinatumomab, achieving MRD-negativity in the blood and bone marrow after the first cycle of therapy. We continued to monitor MRD with clonoSEQ, using a combination of bone marrow and blood samples to enable more frequent monitoring. The patient has now been MRD-negative for 2 years and I continue to use clonoSEQ to monitor his remission.”
Clinician has received compensation to participate in advisory meetings sponsored by Adaptive. Clinician’s research has also been supported, in part, via product grants.
*BLINCYTO® is indicated for the treatment of B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% in adults or children. This indication is approved under accelerated approval based on MRD response rate and hematological relapse-free survival. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials. WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO®. Interrupt or discontinue BLINCYTO® as recommended. Neurological toxicities, which may be severe, life-threatening or fatal, occurred in patients receiving BLINCYTO®. Interrupt or discontinue BLINCYTO® as recommended. Please see full Prescribing Information, including Boxed WARNINGS and Medication Guide, for BLINCYTO®.
** Please see last page for important safety information about blinatumomab. WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO®. Interrupt or discontinue BLINCYTO® as recommended. Neurological toxicities, which may be severe, life-threatening or fatal, occurred in patients receiving BLINCYTO®. Interrupt or discontinue BLINCYTO® as recommended.
Use of the clonoSEQ Assay
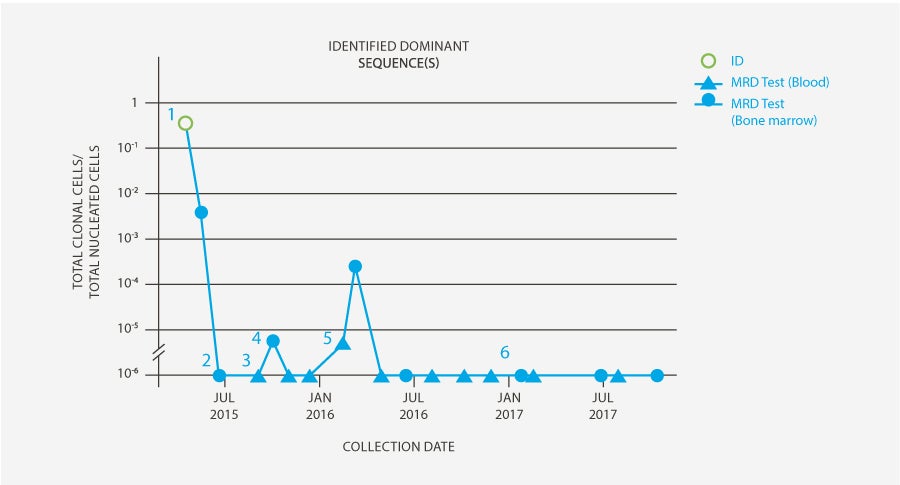
-

clonoSEQ Clonality (ID) test was performed on a high disease load sample to identify DNA sequences for subsequent MRD testing.
-

After consolidation, patient achieved MRD-negative CR by clonoSEQ and proceeded to allogeneic stem cell transplant.
-

Patient tested MRD-negative in the blood 60 days post-transplant.
-

100 days post-transplant, clonoSEQ MRD in bone marrow showed low-level residual disease (5 cells per million). Patient underwent immune suppression taper to maximize graft vs. leukemia effect.
-

Subsequent blood-based MRD monitoring revealed residual disease, prompting an immediate repeat bone marrow biopsy which showed rising MRD (>100 cells per million). Based on these results, blinatumomab was administered in Feb’16 to address residual disease.
-

Following blinatumomab treatment, all peripheral blood and bone marrow samples for this patient have remained MRD-negative by clonoSEQ (as of Jan ’18) at a sensitivity of 1 in 1 million leukemic cells.
Blood-based MRD testing for patients with acute lymphoblastic leukemia is available as a CLIA-regulated laboratory developed test (LDT) service provided by Adaptive Biotechnologies. This use of clonoSEQ has not been approved or cleared by the FDA.
*This case study was based off results generated from an earlier version of the clonoSEQ Assay.
Intended Use:
The clonoSEQ Assay is an in vitro diagnostic that uses multiplex polymerase chain reaction (PCR) and next-generation sequencing (NGS) to identify and quantify rearranged IgH (VDJ), IgH (DJ), IgK and IgL receptor gene sequences, as well as translocated BCL1/IgH (J) and BCL2/IgH (J) sequences in DNA extracted from bone marrow from patients with B-cell acute lymphoblastic leukemia (ALL) or multiple myeloma (MM), and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL).
The clonoSEQ Assay measures minimal residual disease (MRD) to monitor changes in burden of disease during and after treatment. The test is indicated for use by qualified healthcare professionals in accordance with professional guidelines for clinical decision-making and in conjunction with other clinicopathological features.
The clonoSEQ Assay is a single-site assay performed at Adaptive Biotechnologies Corporation in Seattle, Washington.
Special Conditions for Use:
- For in vitro diagnostic use.
- For prescription use only (Rx only).
Limitations:
ALL, MM, and CLL:
MRD values obtained with different assay methods may not be interchangeable due to differences in assay methods and reagent specificity. The results obtained from this assay should always be used in combination with the clinical examination, patient medical history, and other findings. The clonoSEQ Assay is for use with specimens collected in EDTA tubes. Results may vary according to sample time within the course of disease or by sampling site location. The assay may overestimate MRD frequencies near the limit of detection (LoD). The MRD frequency LoD varies based on the amount of gDNA that is tested and using lower gDNA input may prevent MRD detection at low frequencies. Sample processing and cell enrichment strategies may affect the measured MRD frequency. The volume and cellularity of sampled input material may affect the ability to detect low levels of disease. False positive or false negative results may occur for reasons including, but not limited to: contamination; technical and/or biological factors such as the type of rearrangement or the size of the junction region. The assay has been validated with the Illumina NextSeq500 and 550.
For CLL:
MRD is based on measurements of tumor cells detected in peripheral blood and/or bone marrow. However, patients may have significant residual disease in unassessed compartments and U-MRD in one compartment cannot fully rule out the presence of disease in the other compartment, for example, U-MRD in blood may not be the same in bone marrow. Therefore assessment of MRD in CLL should employ a multimodal approach including clinical examination, patient medical history, and other findings. Outcome for patients with MRD detectable in bone marrow but not in peripheral blood (PB-/BM+) may differ according to type of therapy. This assay is capable of monitoring specific tumor clonotypes. The association between MRD assessments and patient clinical status for the purpose of monitoring changes in disease (e.g., relapse, remission, stable disease) has not been demonstrated. The value of MRD in CLL for previously untreated or “watch and wait” patients is not established. CLL is a heterogeneous disease. MRD values and expectations for outcome may not be generalizable across treatments. Changes in MRD should be interpreted with caution when used to evaluate disease burden in therapies that have not been validated. Regardless of MRD status, cytogenetics play an independent role in patient risk status and its impact on PFS/OS.
For important information about the FDA-cleared uses of clonoSEQ including test limitations, please visit clonoSEQ.com/technical-summary.
 Back
Back